
Keep in mind to start with the electronegative atoms and proceed to the electropositive one. Fillup the octet of the atoms with the remaining electrons.Draw a skeletal structure with single bonds only.Choose a central atom generally the atom with the highest bonding sites.Do take care of +, – signs while calculating. Calculate the total number of valence electrons in the molecule.Have a look at the steps jotted down below:. There is a common way by which we can draw the lewis structure of any compound.
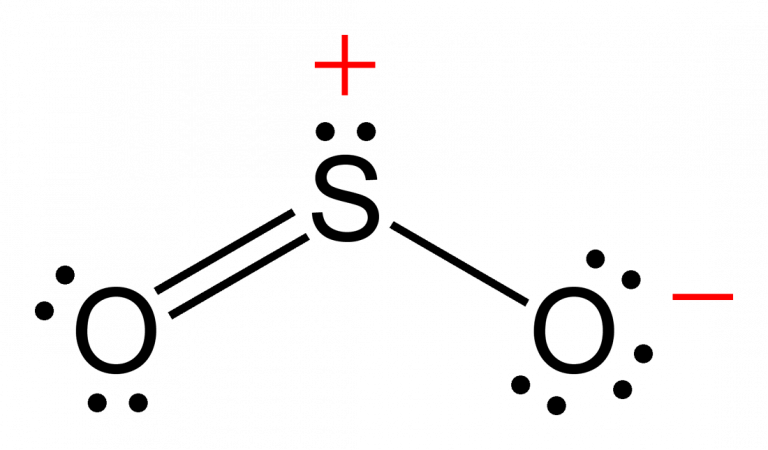
ELECTRON DOMAIN GEOMETRY OF SO2 HOW TO
How to draw a lewis structureĪ lewis structure helps us to find out about the structure of the compound, types, and the number of bonds, physical properties, and how the compound interacts with other compounds.ĭrawing a lewis structure is pretty simple! So let’s move on to these parts one by one in detail.īefore going into the lewis structure of nitrous oxide, it’s better to know the steps to draw a lewis structure. Now to understand every other reaction involving N2O, we need to know about its lewis structure, hybridization, and bonding. Moreover, nitrification and denitrification are two biological or natural processes that can produce nitrous oxide. Along with these, nitrous oxide is also a major component of the earth’s atmosphere. There are many more reactions that are used for the preparation of N2O. This process is known as the Ostwald process. Ostwald process:- Oxidation of ammonia with manganese dioxide and with bismuth oxide as catalyst gives us nitrous oxide. Heating the mixture of sodium nitrate and ammonium sulfate gives us N2OĢ NaNO3 + (NH4)2SO4 -> Na2SO4 + 2N2O + 4 H2OĪlso, nitrous oxide can be formed by reacting urea, nitric acid, and sulfuric acid,Ģ (NH2)2CO + 2 HNO3 + H2SO4 → 2 N2O + 2 CO2 + (NH4)2SO4 + 2H2O Laboratory methods:- Preparation of nitrous oxide can be done in the lab as well. Industrial methods:- On an industrial scale, heating of ammonium nitrate gives us nitrous oxide and water vapor. 6 + (3 x 6) = 24.Nitrous oxide can be prepared in more than one way. That means SO 3 has 24 valence electrons. Sulfur brings 6, and oxygen brings 3 each. Valence: Here, sulfur in the center because of its lowest electron capability, and three oxygen around it. (By the way, that is the reason why SO 3 is having the shape of Trigonal Planar.) The bond angle of SO 3 is 120 degrees. That means we have AX3 for the SO 3 molecule. Moreover, as there are three oxygen, it will be X3. In this formula of SO 3, we don’t have any non-bonding electron, and that is why we don’t bother about N. N stands for any nonbonding electron pairs.
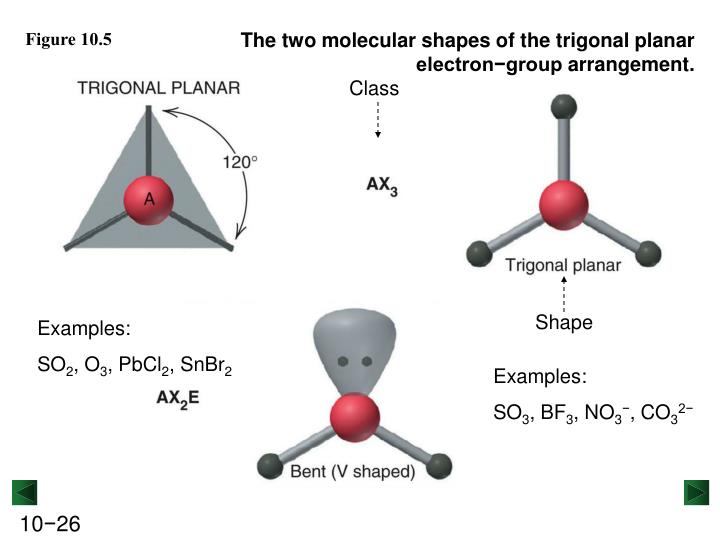
These electrons are negative and repel each other. There are one sulfur atom and three oxygen atoms which are spread out as far away as they can! Atoms of oxygen are surrounded by electrons. SO 3 includes two components mainly – Sulfur and Oxygen. It can determine reactivity, polarity, color, attraction, biological activity, etc. Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule. SO3 Hybridization Sulfur Trioxide Molecular Geometryīeing an intelligent and well-practiced human being, you must know what is molecular geometry, but let me revise it for the all young students out there.


 0 kommentar(er)
0 kommentar(er)
